Patients
-
- Angiography
- Angioplasty and Stenting
- Aortic Aneurysms
- Biliary Drainage and Stenting
- Carotid Artery Stenting
- Central Venous Access
- Colonic Stenting
- Fibroids
- Gastrointestinal Haemorrhage
- Gastrostomy
- Hepatic Malignancies
- Kidney Tumour Ablation
- Minimally Invasive Treatments for Vascular Disease
- Nephrostomy
- Oesophageal Stents
- Pelvic Venous Congestion Syndrome
- Percutaneous Nephrolithotomy
- Prostate Artery Embolisation PAE
- Pulmonary Arteriovenous Malformations
- PAE Patient Information Leaflet
- Ureteric Stenting
- Varicoceles
- Varicose Veins
- Vascular Malformations
- Vertebral Compression Fractures
- Vertebroplasty and Kyphoplasty
Vascular Malformations
What are vascular anomalies?
Vascular anomalies are a diffuse collection of vascular abnormalities that are present at birth or soon after. Historically the terminology has been complicated, descriptive, difficult to understand and a barrier to communication between specialities. The International Society for the Study of Vascular Anomalies (ISSVA) was founded after 16 years of biennial meetings with the primary goal of improving the understanding of these abnormalities. ISSVA adopted the biological classification system developed by Mulliken & Glowacki published in 1982 (see below). This classification differentiated vascular anomalies due to their endothelial cell characteristics and clinical behaviour. It simply distinguished two main types of vascular anomalies: vascular tumours (grow by cellular mainly endothelial hyperplasia) and vascular malformations (localised defects in vascular morphogenesis caused by dysfunction in embryogenesis and vasculogenesis). This simplified classification helps clinicians decide the appropriate treatment algorithm.
What are vascular malformations?
Vascular malformations (VMs) have a quiescent endothelium (ie: inner lining) and are considered defects of vascular morphogenesis. The majority are therefore present at birth, although not necessarily clinically apparent, and persist throughout life. Occasionally VMs can be acquired; these are most commonly high flow and relate to trauma, either blunt or penetrating. They can also be iatrogenic. VMs are typically subdivided by their cell type and flow characteristics (see below)
|
Vascular Malformations |
|
|
Low Flow |
High Flow |
|
Venous Malformations (VM) Lymphatic Malformations (LM) Mixed Malformations
|
Arteriovenous Malformations (AVM) |
What are arterio-venous malformations (AVMs)?
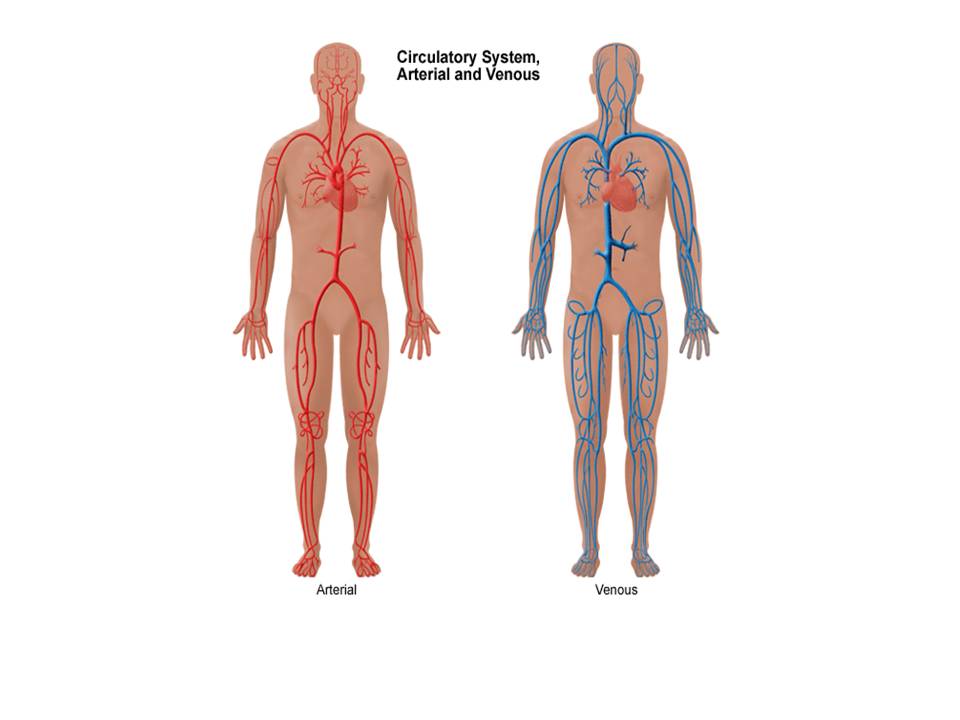 AVMs are defects of the circulatory system that can arise during embryonic or foetal development or can be acquired, as described, at any stage after birth.
AVMs are defects of the circulatory system that can arise during embryonic or foetal development or can be acquired, as described, at any stage after birth.
In the normal situation, blood vessels called ‘arteries’ carry oxygenated blood away from the heart to all tissues and organs of the body. Inside each organ or tissue these arteries divide into smaller microscopic vessels called capillaries. It is at this level that oxygen is transferred from the blood to the tissues. Deoxygenated blood is then carried away from the tissues and back to the heart and lungs via blood vessels called ‘veins’ (see diagrams).
Diagram showing arteries and veins of the body
In a normal situation blood in arteries is under high pressure and blood in veins is under low pressure. The pressure reduction occurs as the blood passes through the capillary network in the tissues and organs.
An AVM is an abnormal connection (or usually multiple small connections) between an artery and vein. The point of abnormal connection between the artery and vein is referred to as the “nidus”. In this situation blood bypasses the capillary network within the organs and tissues and the normal pressure down regulation does not occur.
Types of AVM
Most AVMs occur spontaneously in-utero due to defects in vasculogenesis, however, some AVMs can be hereditary (e.g. hereditary haemorrhagic telangectasia) or be acquired (e.g. following trauma).
AVMs are ‘high-flow’ vascular malformations, related to the pressure of blood within them due to the direct communication of the artery and vein. AVMs have also been classified on a variation of spinal AVMs developed by Houdart in 1992. This classification separates high flow AVMs into 3 types dependent on the number of arterial and venous communications and the number of nidi present in the malformation.
|
Houdart Classification |
|
|
|
Type 1 |
Arteriovenous |
No more than 3 separate arteries. |
|
Type 2 |
Arteriolovenous |
Multiple arteries shunt to a single vein. |
|
Type 3 |
Arteriolovenulous |
Multiple shunts between arteries and venules. |
What are the symptoms of an AVM?
This depends largely on the site of the AVM and symptoms can vary significantly. The majority of AVMs however can be asymptomatic until a complication occurs. The most common and potentially life-threatening complication of AVMs is bleeding. Symptoms are often non-specific and can be caused by a wide range of other medical conditions.
AVMs in the brain may present with headaches, seizures or weakness of one or more parts of the body, or may only become apparent after a bleed (ie: burst / rupture).
AVMs in the lungs are typically asymptomatic although shortness of breath on exertion and the coughing up of blood (haemoptysis) can occur.
AVMs in the gastrointestinal tract typically present with bleeding and abdominal pain. The bleeding may be minimal and manifests itself as black stools or even anaemia (low blood count).
AVMs in the soft tissues (for example on the face, trunk, arms or legs) may typically be cosmetic, seen as a swelling or asymmetry that may or may not be painful. On the trunk and limbs these are often lumps that can be painful under certain circumstances and can over a period of time be seen to grow. Occasionally these AVMs can be disfiguring. Most soft tissue lumps are often thought to be tumours and sent for a biopsy before the diagnosis of an AVM is made.
AVMs may also show rapid growth in size over a relatively short time period or swelling may develop in the months following localised trauma if this is the cause. They can be classified related to their symptoms using the Schobinger classification, which can help plan the timing of treatment / intervention.
|
Schobinger Classification |
|
|
Type 1 |
Quiescent - stable |
|
Type 2 |
Growing |
|
Type 3 |
Symptomatic: pain, bleeding or functional problems |
|
Type 4 |
Decompensating, high output cardiac failure |
If AVMs are left untreated they may progress with growth, bleeding and eventually heart failure due to the rapid transit of blood through the AVM bypassing the rest of the body and returning rapidly to the heart. This leads to the hearts inability to cope with the increased blood flow and may lead to shortness of breath or chest pain and light-headedness.
Can AVMs be prevented?
At present very little can be done to prevent the development of an AVM. However, their progression and complications can be prevented if tailored treatment is appropriately offered. Life-threatening situations can be prevented from arising and stop the AVM getting larger.
How are AVMs diagnosed?
Your doctor will have a suspicion or a good indication that you have an AVM on the basis of the history you provide and on physical examination or in some circumstance your family history. Your doctor will refer you to a specialist.
The diagnosis is typically clinical and imaging the lesion is key to understand the size and nature of the lesion. Imaging can also help decide when and how to treat these lesions. Ideally AVMs should be managed by a ‘multi-disciplinary’ team of doctors, nurses and specialists with a specific interest and experience in diagnosing and treating this condition.
Ultrasound: This is a test that uses sound waves to detect abnormalities and is particularly good at showing flow of blood as it a dynamic and non-invasive investigation. This test will help to determine the flow characteristics of the lesion but will not give a detailed anatomical picture of the problem.
CT scan: This a non-invasive X-ray test that allows the radiologist to see the body in slices on a computer screen. An injection of special ‘dye’ called ‘contrast’ is used at the same time to highlight blood vessels and this is sometimes called a CT Angiogram. These images can then be manipulated on a computer and give a detailed 3-D picture of the AVM and allow management planning.
MRIscan: This is similar to a CT scan but uses magnetic fields instead of radiation. Often MRI gives more detailed anatomical information especially in the brain.
Figure: MRA demonstrating abnormality around the left elbow consistent with a high flow AVM.
Angiography: This is a more invasive investigation that involves the introduction of a small, narrow plastic tube into the blood vessels called a catheter. This is usually carried out using local anaesthetic. The catheter is placed typically in the artery in the groin called the femoral artery. A picture of the blood vessels can be obtained by injecting dye (contrast) through the catheter using an X ray machine. This gives detailed information to the nature and anatomy of the blood vessels making up the AVM. This test is not always necessary nowadays as CT and MRI scanning offers similar accuracy. Angiography is usually carried out at the same time as treatment or occasionally if CT or MRI findings are indeterminate.
Figure: Digital subtraction angiogram of a hand. It shows an abnormality (red arrow) in keeping with a high flow AVM which was situated in the palm of the hand.
How can AVMs be treated?
AVMs are very complicated. It is important that treatment and follow up is carried out and supervised by a multi-disciplinary team with an interest in vascular malformations. The management of these conditions requires input from the whole team.
Most AVMs do not require immediate treatment and often surveillance is all that is required (Schobinger type 1). Even those that are growing (Schobinger type 2) can be actively monitored. The decision to treat AVMs depends on a number of factors including presenting features, site, size and symptoms. An AVM that presents with acute bleeding may require emergency treatment but often an elective approach with multidisciplinary input is best for the patient.
The most important factor is that each AVM is addressed on an individual basis taking into account the above factors and most importantly detailed discussion with the patient.
AVMs have historically been difficult entities to manage. Surgery has been the mainstay for decades with a number of different techniques employed. Recent advances in the understanding of AVMs and the technological developments within Interventional Radiology successful minimal invasive treatments are preferred. Surgery still has a role in specialist centres in collaboration with the Interventional Radiologists available.
Treatment options range from endovascular therapy to surgery to radiation treatment. Endovascular therapy is the treatment of AVMs using catheters and needles placed in the blood vessels under x-ray control to allow the permanent blockage of the abnormal communications. This can be achieved by a number of techniques, which are known as embolisation. These techniques are carried out by Interventional Radiologists and are usually the safer option compared to surgery.
Embolisation Treatment
This is defined as the intentional occlusion of the blood vessels that make up the AVM. This is carried out in conjunction with an angiogram (see earlier). Depending on the embolisation agent and access technique used by the Interventional Radiologists a general anaesthetic may be required.
Embolisation of these lesions typically involves one of two techniques:
- Percutaneous direct stick and direct instillation of embolic material into the nidus of the AVM.
- Endovascular transcatheter embolisation. This is very similar to the angiogram you will have had but often involves using more specialised catheters and guidewires. The embolic material is then delivered via the catheter. An arterial or venous approach can be used.
There are a number of embolic materials used. The interventional radiologist will discuss these with you. They range from liquids (sodium tetradecyl sulphate STD, alcohol, histoacryl glue and Onyx™), metal springs (coils) or metal plugs that block the blood vessels in this region.
.jpg) Treatment can be curative, but more often is palliative, in that it is to reduce potentially life threatening complications and down grade the AVM. Simple AVMs maybe treatment successfully in a single session but the majority require more than one treatment session is required over a period of time.
Treatment can be curative, but more often is palliative, in that it is to reduce potentially life threatening complications and down grade the AVM. Simple AVMs maybe treatment successfully in a single session but the majority require more than one treatment session is required over a period of time.
Figure: Traumatic AVM of the lip. Angiogram demonstrating the typical appearances treated with Onyx embolisation and the resultant cessation of blood flow.
Surgery
Surgical resection of AVMs can be appropriate is some cases but is fraught with danger as these lesions can bleed significantly at surgery. Surgery can also be quite disfiguring and even lead to functional impairment. AVMs can often recur after surgery.
That’s not to say there is no place for surgery in the management of AVMs. Often surgery and embolisation are complimentary where the lesion is first de-vascularised (blood supply reduced) by embolisation and then surgery performed to remove the residual AVM with limited blood loss at the time of the operation.
Radiation Treatment
Radiosurgery utilises a focal beam of radiation, directing it towards the AVM. This beam destroys the walls of the blood vessels making up the AVM and the lesion gradually shrinks. This type of treatment is only currently used in AVMs in the brain and is not widely available in the UK.
Are there any complications to the treatment of AVMs?
As with all medical treatments complications can arise. In AVM treatment complications can be broken down into those related to the angiographic procedure and those related to the embolisation or surgical procedure itself.
The angiographic complications are covered in a separate section on the website, but in summary include a very small risk of bleeding and bruising at the puncture site and infection along with a small risk of damaging the blood vessels as the catheter is advanced.
Complications related to the embolisation procedure are related to the delivery of the embolic agent and its local and systemic effects. Pain is often encountered following the procedure but this is usually short-lived and may last a few days. This can be controlled with pain-killers of different strengths depending on the degree of pain. Swelling is sometimes experienced at the site of treatment and is related to the local inflammatory process caused by blocking the blood vessels and sometimes by the embolic agent itself. Occasionally nausea can be encountered but this is well controlled with anti-sickness medication.
One of the main risks of AVM embolisation is ‘non-target’ embolisation. This is when the embolic agent passes in to the wrong blood vessels at the time of delivery with or without causing problems in this blood vessel. Even if some material passes into the wrong vessel there is an even smaller chance that it may cause problems. This risk varies in each individual patient and depends on a number of factors but is generally less than 5%. However this risk will rise with the complexity of the AVM.
The risks will be discussed with you prior to any procedure but are generally low as embolisation is proven as a safe and effective method of treating AVMs worldwide.
What are venous malformations?
Venous malformations are abnormally developed blood vessels with varying degrees of communication with normal veins. They are sometimes described abnormal ‘vascular lakes’ containing very slow moving blood. Supporting these vascular lakes is a solid component known as matrix. The ratio of spaces and matrix within a vascular malformation varies considerably from patient to patient. It can differ to some extent within different malformations within the same person. They are classified as low flow lesions meaning that they contain venous blood, which is very slow moving. Occasionally the blood moves so slowly that the blood can clot within the malformation. This usually leads to swelling and pain within the malformation.
They can occur anywhere in the body and are present at birth, although they may not become apparent until later in life. Other situations when they may be come apparent are following episodes of local trauma, at puberty or pregnancy due to hormonal changes occurring at these times. Depending on their location venous malformations may cause pain, swelling, restriction of movement or cosmetic issues. Occasionally venous malformations can bleed especially if they are in a very superficial position. Treatment may be indicated because of the appearance or for associated functional problems.
Types of venous malformations
VMs have a wide range of appearances on imaging with ultrasound, Magnetic resonance or venography. They can range in the amount of matrix present, size of the vascular spaces and communication between spaces as well as the drainage network into the main venous system. There is no universally accepted classification system for low flow venous malformations; Kok et al in 2010 described a classification system based on the tissues involved by the low flow VM stratify risk & treatment outcomes (see below):
|
Birmingham Classification |
|
||
|
|
Limbs |
Head & neck |
Trunk |
|
Type 1
|
Superficial (skin and subcutaneous tissue) (a) Localised (b) Diffused |
||
|
Type 2 |
Fascia/muscle involvement |
Fascia/muscle/mucosa involvement |
Fascia/muscle involvement |
|
Type 3 |
Bone/joint involvement |
Bone/joint/airway involvement |
Spinal/central nervous involvement |
|
Type 4 |
Diffuse whole limb involvement +/- hypertrophy (eg. Klippel-Trenaunay) |
Ocular/intracranial involvement |
Intraperitoneal involvement |
Table: Low flow VM Birmingham classification
Puig et al in 2003 described a classification system based on the venous drainage pattern of the low flow VMs to stratify risk related to treatment of these lesions (see below)
|
Puig classification |
|
|
|
Description |
|
Type 1 |
Isolated malformation without peripheral drainage |
|
Type 2 |
Malformation that drains into normal veins |
|
Type 3 |
Malformation that drains into dysplastic veins |
|
Type 4 |
Malformation that represents a dysplasia |
Table: Puig classification for low flow VM drainage patterns
What are the symptoms of venous malformations?
Typically the main symptoms encountered include pain and swelling. The swelling is typically dependent; by lowering the area affected to below the heart allows the lesion to swell. A significant number of venous malformations involve the skin giving a bluish discolouration. The bluish discolouration and swelling can lead to cosmetic issues depending on the site of the lesion. Intermittently the venous malformation can become painful, swollen and hard. This is mainly due to episodes of thrombosis (blood clot) within the lesion itself. These blood clots typically do not move to the lungs in the majority of circumstances.
Can venous malformations be prevented?
At present there is nothing that can be done to prevent the development of a venous malformation but there are several forms of treatment available. These types of lesions usually occur spontaneously and are not inherited and cannot be passed on to children. There are several syndromes that can have a familial tendency but these are extremely rare.
How are venous malformations diagnosed?
Your doctor will have a suspicion or a good indication that you have an AVM on the basis of the history you provide and on physical examination or in some circumstance your family history. Your doctor will refer you to a specialist.
The diagnosis is typically clinical and imaging the lesion is key to understand the size and nature of the lesion. Imaging can also help decide when and how to treat these lesions. Ideally VMs should be managed by a ‘multi-disciplinary’ team of doctors, nurses and specialists with a specific interest and experience in diagnosing and treating this condition. The most useful imaging for low flow VMs is ultrasound and MRI, however occasionally a CT has been performed. The appearance of venous malformations are usually typical on MRI, however occasionally can look different and a biopsy may be required to confirm the diagnosis and exclude other more sinister causes that may present similarly to venous malformations.
.jpg)
Ultrasound: Is a very useful imaging test that can be performed in clinic to assess the VM, especially its morphology of spaces to matrix. It is very helpful in assessing suitability for treatment.
Figure: Two ultrasound pictures of a low flow VM demonstrating vascular spaces and a calcified nodule called a phlebolith.
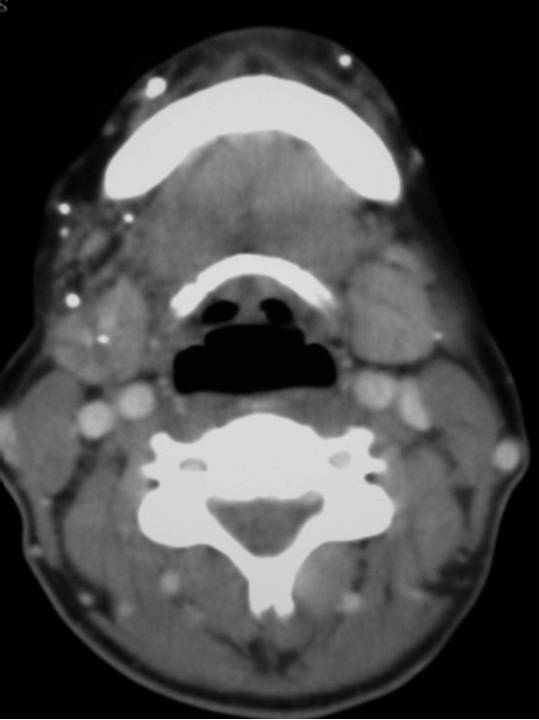 CT Scan: This is not routinely performed but can demonstrate the presence of multiple calcified foci consistent with phleboliths commonly seen in low flow VMs.
CT Scan: This is not routinely performed but can demonstrate the presence of multiple calcified foci consistent with phleboliths commonly seen in low flow VMs.
Figure: Extensive low flow VM.
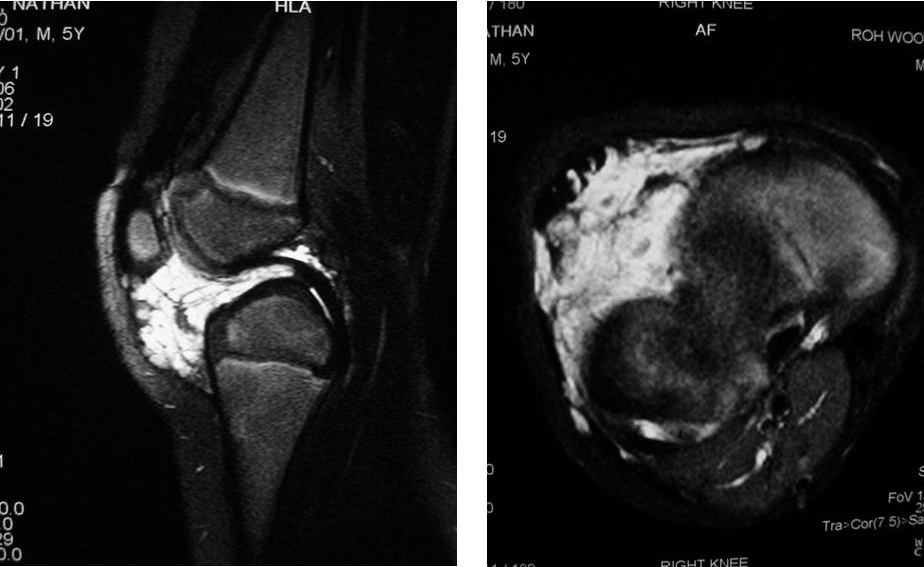 MRI scan: This is the most useful imaging for low flow VMs. They are often known as “iceberg lesions” in that what you see is only the tip. MRI is able to demonstrate the total extent of the underlying malformation.
MRI scan: This is the most useful imaging for low flow VMs. They are often known as “iceberg lesions” in that what you see is only the tip. MRI is able to demonstrate the total extent of the underlying malformation.
Figure: Fat saturated T2 saggital & axial image VM affecting the right knee
How can venous malformations be treated?
Treatment options are divided up in to conservative management, percutaneous injections or surgery or a combination of these. If there are no symptoms then there is no indication or need for treatment.
Initially it important to determine the exact symptoms and to what degree this is distressing the patient and how much of an impact this is having on their life. The majority of venous malformations do not need treatment, but this can be reviewed at any time, especially if symptoms worsen or change. Venous malformations are not malignant and do not have malignant potential.
Conservative management includes pain relief with anti-inflammatory tablets, compression dressings if the lesion is in a limb and alteration of lifestyle accordingly. This approach is usually advised if symptoms are well tolerated.
Treatment depends on the number of vascular spaces within the lesion and the amount of more solid tissue. Lesions with a more spacious component (ie: more venous lakes) are more suitable for injection therapy or ‘sclerotherapy’ than those that are predominantly solid in nature. Depending on the site, size amongst other factors certain lesions are suitable for surgical removal.
Treatment options will be discussed in the multidisciplinary clinic with the patients’ opinion taken into account.
What is ‘sclerotherapy’?
Sclerotherapy is a type of treatment that involves the injection of a special chemical into the venous malformation to ultimately shrink it and relieve the symptoms it is causing. Various substances can be used but most commonly the chemical used is Sodium Tetradecyl sulphate (Fibrovein). When injected into a lesion it causes an inflammatory reaction which leads to localised blood clots and the formation of a scar in place of the venous malformation. This corresponds to shrinkage of the malformation. This is carried out under ultrasound and x-ray control as the doctor needs to be sure that the correct part of the malformation is accessed with the needle and needs to assess the degree of communication with adjacent communicating veins.
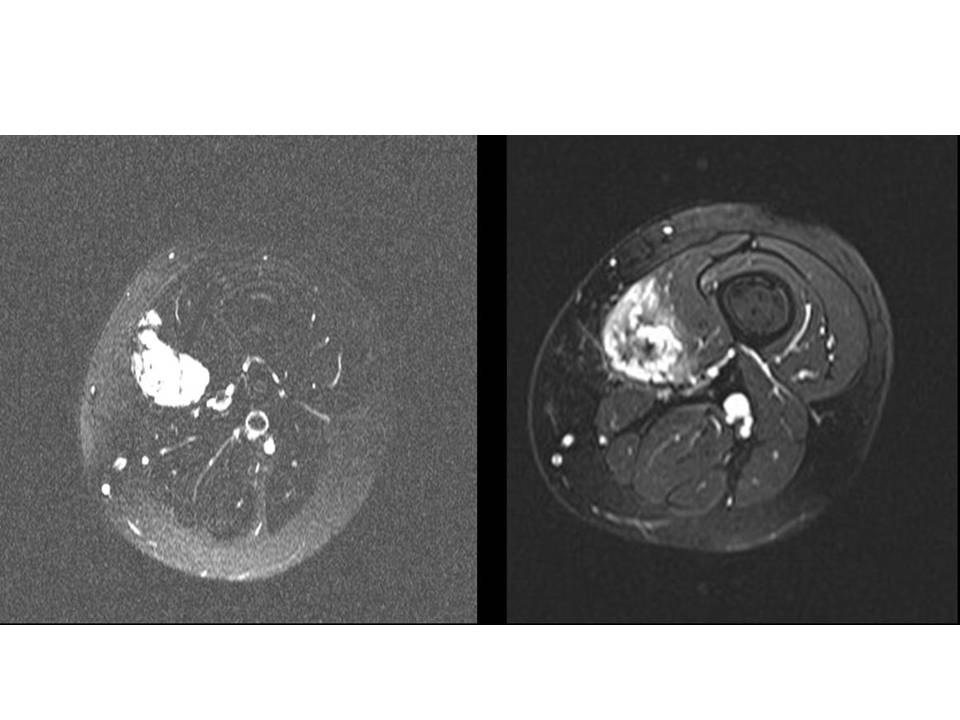 Often a ‘course’ of multiple injections are required to adequately treat a venous malformation and it can be some time before the patient notices a significant difference. Not all venous malformations are successfully treated in this way but in the vast majority of cases significant results are achieved.
Often a ‘course’ of multiple injections are required to adequately treat a venous malformation and it can be some time before the patient notices a significant difference. Not all venous malformations are successfully treated in this way but in the vast majority of cases significant results are achieved.
Sclerotherapy is not a ‘cure’ for these malformations but is aimed at symptom control and reduction in size. Sclerotherapy may not treat skin discolouration associated with some malformations. It is typically carried out as a day case procedure in hospital.
Figure: MRI scans through the thigh. The left hand image shows a VM before treatment .The right shows the VM after sclerotherapy 6 months later. It is no not as bright and has a central dark area consistent with fibrosis.
What are the risks of sclerotherapy?
Immediately after the injection considerable swelling can occur along with pain. This usually settles within days – weeks of the injection. Pain is usually adequately treated with oral pain killers. It is when this swelling settles that there is a noticeable reduction in the size and / or symptoms of the malformation.
There is a small risk of infection and bleeding but as sclerotherapy is carried out via a needle puncture and not an incision this is unusual.
There is a small chance that the skin over the malformation will breakdown and lead to a blister or even a small ulcer, but this will improve in time and possibly leave a scar. This is more common in superficial malformations that involve the skin or occupy a large area directly under the skin (subcutaneous). Surgical revision of sclerotherapy induced scars is an option should this be required.
If a malformation is near a nerve or group of nerves the swelling induced by sclerotherapy can sometime compress the nerve and stun it. This can lead to loss of sensation in the area or even local muscle weakness; this can for example occur in facial lesions but is uncommon. This condition is termed ‘neuropraxia’ and if it occurs at all is often temporary and is rarely permanent.
General anaesthetic is often required for sclerotherapy and this carries a small risk.
These are the general risks and the multidisciplinary team will discuss more specific risks to an individual according to the size, site and type of malformation.
What are ‘Lymphatic Malformations’?
Lymphatic malformations are again within the spectrum of vascular anomalies. They are often large fluid filled spaces containing lymph as opposed to blood like venous malformations. The other type of lymphatic malformation is ‘microcystic’ and this type contains multiple tiny spaces.
The macrocystic malformations are commonly diagnosed in childhood and can grow to be very large. They can occasionally compress adjacent structures and if these are deemed important structure like for example the trachea (windpipe), treatment may be required soon.
They are treated in a similar fashion to venous malformations (ie: sclerotherapy) and similar risks are present. If the malformation is near the trachea then often a short hospital stay is required as the swelling induced by sclerotherapy can compress the trachea. This risk of this is minimal in most cases but this is discussed with the patient (or parents) prior to the procedure.
Microcystic lymphatic malformations can be more challenging to treat. There is a drug called bleomycin which has been used successfully to treat these malformations. This is a well established chemotherapy agent which is used to treat certain types of cancer (although lymphatic malformations are not cancer) and its use in much smaller doses can improve the appearance and symptoms of microcystic lymphatic malformations with excellent results. It can also be used in the treatment of other vascular anomalies.
What is Bleomycin treatment for vascular anomalies ?
Bleomycin is widely used as a chemotherapy agent to treat certain types of cancer. Vascular anomalies are not cancers but bleomycin has been shown to be effective in treating certain types of vascular anomalies when injected directly into the lesion, rather than into the bloodstream as with chemotherapy. Subtypes of vascular anomalies successfully treated include, lymphatic malformations (macro and microcystic) and even venous malformations. Bleomycin works by exerting its effect on the lining of the malformation and preventing further growth and promoting regression. It does this by inhibiting local DNA synthesis. Results are encouraging with its use. This form of treatment is not widely available and often requires a course of injections over a period of months. Bleomycin treatment is considered in a multi-disciplinary setting following discussion with the patient and review of cross-sectional imaging. It can be considered as a first line treatment in micro-cystic lymphatic disease.
What are the Risks of bleomycin treatment ?
The risks are similar to conventional sclerotherapy in that pain and swelling are the commonest post-procedural occurrences. Occasionally ulceration can occur, especially when the lesion is close to the skin (or lining of mouth). Flu-like symptoms have also been described and usually only last for a day or so. When bleomycin is used in much higher doses and intravenously to treat cancers there is a small risk of causing lung damage. Specifically lung fibrosis has been described but when used intra-lesionally in the setting of vascular anomalies this risk is extremely small and there are no reported cases in the world. With this in mind a full assessment of lung function is carried out prior to and during a course of bleomycin treatment. If there is any suggestion of pre-existing lung problems bleomycin will not be administered.
The risks are similar to conventional sclerotherapy in that pain and swelling are the commonest post-procedural occurrences. Occasionally ulceration can occur, especially when the lesion is close to the skin (or lining of mouth). Flu-like symptoms have also been described and usually only last for a day or so. When bleomycin is used in much higher doses and intravenously to treat cancers there is a small risk of causing lung damage. Specifically lung fibrosis has been described but when used intra-lesionally in the setting of vascular anomalies this risk is extremely small and there are no reported cases in the world. With this in mind a full assessment of lung function is carried out prior to and during a course of bleomycin treatment. If there is any suggestion of pre-existing lung problems bleomycin will not be administered.
