Patients
-
- Angiography
- Angioplasty and Stenting
- Aortic Aneurysms
- Biliary Drainage and Stenting
- Carotid Artery Stenting
- Central Venous Access
- Colonic Stenting
- Fibroids
- Gastrointestinal Haemorrhage
- Gastrostomy
- Hepatic Malignancies
- Kidney Tumour Ablation
- Minimally Invasive Treatments for Vascular Disease
- Nephrostomy
- Oesophageal Stents
- Pelvic Venous Congestion Syndrome
- Percutaneous Nephrolithotomy
- Prostate Artery Embolisation PAE
- Pulmonary Arteriovenous Malformations
- PAE Patient Information Leaflet
- Ureteric Stenting
- Varicoceles
- Varicose Veins
- Vascular Malformations
- Vertebral Compression Fractures
- Vertebroplasty and Kyphoplasty
Hepatic Malignancies
Interventional radiology techniques in hepatic malignancies
Dr Raj Das, Dr Graham Munneke, Dr Andrew Hatrick
(Please note that some of the illustrations for this section have yet to be uploaded)
What are they, and how can they be treated?
Interventional radiological techniques can offer patients primary cancer treatments or relief from symptoms using minimally-invasive treatments. It is now a relatively frequent occurrence that patients with cancer will come into contact with an interventional radiology department.
The number of procedures available is increasing rapidly and range from direct treatment of the primary tumour by ablation (direct energy applied to a tumour to cause cell destruction) or chemoembolisation (delivery of chemotherapy drugs directly to the tumour), to the relief of obstruction using stents. Interventional radiologists are also often involved in the placement of long-term venous access catheters to aid delivery of other treatments, and drainage of fluid collections for symptom control.
Minimally invasive techniques can be used to diagnose and biopsy lesions; treat primary or secondary malignancies using catheter based delivery of chemotherapy (chemoembolisation or selective internal radiation therapy (SIRT)) or percutaneous ablation techniques (using radiofrequency ablation (RFA) in liver, renal and lung tumours).
It is increasingly possible to treat complications arising from malignant disease using stents to open blocked organs (such as in obstruction of the bile ducts, kidneys and ureters, and superior vena cava), and embolization to stop haemorrhage. Advances in technology, gene-based therapies and new methods of ablation will dictate future interventional oncology treatments.
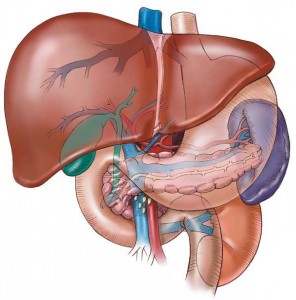
Liver anatomy
An adult human liver normally weighs between 1.4-1.6 kg (3.1-3.5 lb), and is a soft, pinkish-brown, triangular organ. Averaging about the size of a rugby ball in adults, it is both the largest internal organ and the largest gland in the human body (not considering the skin).
It is located in the right upper quadrant of the abdominal cavity, resting just below the diaphragm. The liver lies to the right of the stomach and overlies the gallbladder.
Figure 1: Anatomy and relations of the liver
[From: www.audiolivres.org/category/liver-disease/]
The liver receives blood from the hepatic artery and portal vein. The hepatic artery carries arterial blood, whereas the portal vein drains venous blood from the intestines and other parts of the abdomen. Just over 70% of hepatic blood flow is supplied by the portal vein, but venous blood is only 80% saturated with oxygen. The portal venous blood therefore supplies only 50-60% of the hepatic oxygen requirement. The remaining oxygen is supplied by hepatic arterial blood, which accounts for 25% of the flow. This is an important factor that assists the technique of chemoembolisation.
Venous drainage from the liver is via the hepatic veins to the inferior vena cava (IVC). The hepatic veins run upward through the liver to the IVC.
Types of Liver tumour
Interventional radiological techniques have had an established role in the treatment of liver malignancies for several years. The liver is a relatively common site for the development of primary cancers (hepatocellular carcinoma or HCC), and for the secondary spread of cancers from another site (often from colorectal cancers or other cancers in the body.)
Primary liver tumours
The majority of primary liver tumours are hepatocellular carcinomas (HCC), with a smaller subset of other types of cancers such as cholangiocarcinomas (cancer arising from the bile ducts.)
Incidence of HCC is highest in Asia and sub-Saharan Africa with as many as 120 cases per 100,000. [1] It is relatively uncommon in Europe and North America. In the UK there are about 1,500 deaths per year from HCC. [2]
Although anyone can develop HCC certain risk factors are associated with its development such as: liver cirrhosis (the effect of longstanding liver disease leading to replacement of liver tissue by scar tissue and regenerative nodules); alcohol use; hepatitis B and C infection; anabolic steroid use; genetic haemochromatosis and aflatoxin exposure (a toxin from a certain species of fungus.)
The numbers of patients diagnosed with HCC worldwide parallels those with viral hepatitis and the majority of cases are associated with hepatitis B and C viruses (HBV / HCV). It is thought that HCC may be diagnosed more frequently in the UK over the next few years due to the hepatitis C epidemic and that there may be a “lag” phase approximately 20–30 years after the rise of this infection in certain populations. [3]
In the UK, up to 40% of cases present with HCC as the first indication of underlying liver disease, in comparison to countries such as Japan where 80% of HCCs are detected by screening of patients with known cirrhosis. [4]
Secondary liver tumours
The liver is a common site for spread from other sites in the body, largely due to its dual blood supply from the hepatic artery and the portal vein. In the majority, liver metastases (cancer deposits spread from elsewhere) are multiple and affect both lobes in approximately 77%. Liver metastases are only solitary in approximately 10%. [5]
Symptoms of hepatocellular carcinomas (HCC)
The clinical presentation of HCC tends to be of slow onset and includes symptoms such as fever of unknown origin, abdominal pain, malaise, weight loss and liver enlargement. Jaundice (yellow discoloration of the skin and eyes) is unusual.
Occasionally the clinical presentation of hepatocellular carcinoma (HCC) can be acute and includes bleeding or hepatic rupture.
Liver function tests can be normal. Alpha-fetoprotein (AFP) levels may be elevated because this protein is commonly produced by HCC; however, this is an insensitive marker because AFP levels may be normal in more than one third of patients.
Symptoms of metastatic disease (cancer deposits spread from elsewhere) within the liver
Symptoms of metastatic liver disease may be few, and the extent of liver involvement on images may be surprising, often with little clinical or laboratory evidence suggestive of abnormal liver function.
The only physical sign may be enlargement of the liver, however about 30% of patients with liver metastases (cancer deposits) have a normal-sized liver. With large liver metastases or with tumours that are critically close to the bile ducts, signs of obstructive jaundice may be present and results of liver function tests may be abnormal. Patients may have weight loss with malaise and abdominal enlargement due to liver enlargement, ascites (fluid within the abdominal cavity), or both. [5] The presence of ascites may indicate widespread tumours in the liver, and is regarded as an adverse sign.
How are liver tumours diagnosed?
Diagnosis of HCC or liver metastases
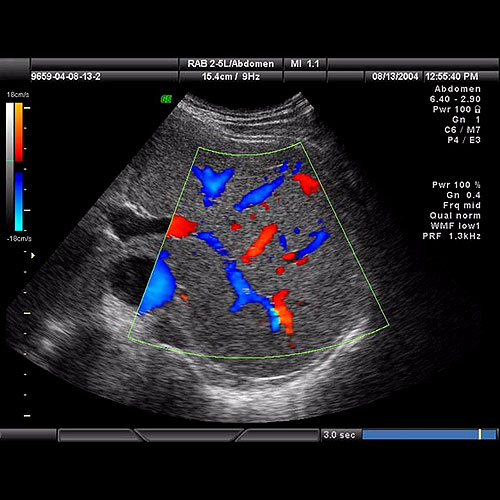
They are commonly diagnosed using ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) scans that are reported by a radiologist. These will provide information about the number, size and characteristics of lesions in the liver.
If there is a question about whether these are malignant (cancerous) lesions then a biopsy may be required which involves the sampling of tissue via the introduction of a fine needle into the liver under ultrasound or CT guidance, under local anaesthetic or intravenous sedation.
Figure 2: Ultrasound image of the liver demonstrating blood vessels (hepatic veins and portal veins) [From: diagnostica.com.my/voluson-730e.php]
Surgical treatments for HCC or secondary liver tumours
The only proven potentially curative therapy for HCC remains surgery, either liver resection or liver transplantation, and patients with a single small HCC (<5 cm) or up to three lesions <3 cm should be considered for assessment for these treatment options.
Surgical resection aims to remove a tumour together with surrounding liver tissue while preserving enough remaining liver for normal body function. This treatment offers the best prognosis for long-term survival, but unfortunately only 10-15% of patients with HCC are suitable for surgical resection. This is often due to extensive disease or poor liver function. Liver surgery in cirrhotic patients carries high morbidity and mortality. The overall recurrence rate after resection is approximately 50-60%.
Liver transplantation may also be considered in any patient with cirrhosis and a small (5 cm or less single nodule or up to three lesions of 3 cm or less) HCC. [3] Liver transplantation aims to replace the diseased liver with a cadaveric liver (from a deceased patient) or a living donor graft. Patients with active hepatitis B virus (HBV) had a worse outlook due to HBV recurrence and were previously not considered candidates for transplantation. Effective antiviral therapy is now available and HBV positive patients with a small HCC, as defined above, can be assessed for transplantation. [3]
Hepatic resection should be considered as primary treatment in any patient with HCC and a non-cirrhotic liver (including fibrolamellar HCC – a specific subtype of HCC that tends to occur in livers without cirrhosis). Resection can be carried out in highly selected patients with cirrhosis and well preserved liver function who are unsuitable for liver transplantation.
Non-surgical treatment of HCC
Non-surgical therapy should be used where surgical therapy is not possible.
Chemoembolisation can produce tumour necrosis (tissue death) and has been shown to affect survival in highly selected patients with good liver reserve. Chemoembolisation is also effective therapy for pain or bleeding from HCC.
Radiofrequency ablation has been shown to produce necrosis (tissue death) of small HCCs. It is best suited to peripheral lesions, less than 3 cm in diameter.
Systemic chemotherapy with standard agents has a poor response rate and should only be offered in the context of trials of novel agents.
Hormonal therapy with Tamoxifen has shown no survival benefit in controlled trials. [3]
New drug therapies that reduce blood vessel growth (angiogenesis) in tumours have been used to some beneficial effect in advanced HCC. Sorafenib (Nexavar, Bayer HealthCare Pharmaceuticals–Onyx Pharmaceuticals) is a newer drug that has been shown to improve survival or time to radiological progression by approximately 3 months, but the drug is not readily available in the UK and is under trial / awaiting further approval. [7]
Interventional radiological (IR) treatment
There are three main IR treatment options:-.
Transarterial chemoembolisation (TACE)
Selective Internal Radiation Therapy (SIRT)
Percutaneous Ablation Techniques / Radiofrequency ablation (RFA)
Transarterial chemoembolisation (TACE)
Embolization is a minimally invasive treatment that occludes, or blocks, one or more blood vessels or vascular channels in the body. In a catheter embolization procedure, medications or synthetic materials called embolic agents are placed through a catheter into a blood vessel to prevent blood flow to the area.
The term “chemoembolisation” refers to the delivery of chemotherapeutic agents with concurrent embolization. Chemo-embolization involves a localized intra-arterial infusion of chemotherapy, emulsified in oil, and combined with a type of embolic material. Transcatheter arterial chemoembolization (TACE) is intended to deliver a highly concentrated dose of chemotherapy to tumour cells, prolonging the contact time between the chemotherapy agents and the cancer cells, and minimizing toxic effects to the rest of the body.
Achieving high, localized concentrations of chemotherapeutic agents within a tumour is the main goal of TACE. The combination of highly concentrated chemotherapy and reducing the blood supply to the tumour is likely to be synergistic in achieving a tumour response. The advantage of chemoembolization over systemic chemotherapy is the achievement of greater concentrations of anti-tumour agents within the tumour. It has been reported that the concentration of chemotherapy within tumour tissue can be up to 100 times higher following chemoembolization than following systemic chemotherapy.[8, 9] Reduced blood flow to the tumour bed prevents washout of the chemotherapeutic agents, [10] resulting in an even greater retention of chemo-therapeutic agents.
History of TACE
First pioneered in the 1970’s, TACE followed after the observation that agents delivered via the hepatic artery, are preferentially taken up by hepatic tumours, whereas normal liver tissue is supplied by the portal venous supply. The liver’s unique dual blood supply (approximately 2/3rd is via portal veins and 1/3rd via the hepatic artery) allows the delivery of treatment and eventually embolisation via the hepatic artery without compromising blood supply to the unaffected liver.
It was over 10 years after the initial development of TACE, that lipiodol (an iodinated contrast agent derived from poppy-seed oil) was found to have a preferential affinity for hepatocellular carcinoma and colorectal metastases and could act as a “delivery system” for chemotherapeutic agents.
Selective Internal Radiation Therapy (SIRT)
What is SIRT?
Both HCC and colorectal metastases are sensitive to radiation therapy (radiotherapy) but the side effects incurred from external beam radiotherapy are generally too high to tolerate. SIRT is the process of intra-arterial injection of millions of microspheres each approximately 35 microns (one-third the diameter of a strand of hair), which are bonded to yttrium-90 (Y-90), a radioactive pure beta emitter with a physical half-life of about two-and-a-half days. The microspheres become trapped in the tumour's blood supply, where they destroy the tumour by reducing blood flow and induce local radiation damage to the cellular DNA of the liver lesion. The radiation is contained within the patient's body, and is continually delivered over approximately two weeks, at which point the microspheres are no longer radioactive.
The spheres are delivered in a similar fashion to TACE via the femoral artery to the hepatic arteries and their branches.
Healthy liver tissue receives most of its blood supply via the portal vein, whilst liver tumours gain the majority of their blood supply from the hepatic artery. Therefore catheterization of the hepatic artery permits the selective targeting of therapeutic material to the tumour.
Technique of SIRT – What to expect in hospital
Unlike chemotherapy, which is administered in repetitive cycles, SIRT is typically given to the patient in one treatment dose (or two doses if each liver lobe is to be treated separately) and patients are normally discharged within 24 hours.
The technique of embolisation is important to prevent reflux of SIRT spheres to the stomach, duodenum and pancreas, which can result in radiation injury to the bowel and ulceration. Vessels that potentially supply these organs, originating from the liver are blocked with metal coils.
The procedure of SIRT is performed in a similar fashion to TACE but using different embolic agents.
All patients should undergo baseline tumour evaluation with CT or MRI to identify tumour anatomy and the presence of complicating factors. The procedure is performed within the interventional radiology suite and under sterile conditions. The technique is performed under sedation allows the patient to feel drowsy. It is not the same as a general anaesthetic, in which the patient is unconscious.
The first part of the procedure involves puncture of the femoral artery (the main artery in the groin) after injection of local anaesthetic. A guidewire is passed up into the aorta (the main artery of the body) and then to selectively catheterise the main arteries to the liver.
A catheter is then advanced super-selectively into the tumour/s and the SIRT yttrium-labelled microspheres are injected into the tumour’s blood supply.
After embolisation, hepatic artery injection of radiolabelled albumin (a protein) is performed and a nuclear medicine lung scan is performed. This is to exclude the presence of significant shunting between the liver and lung circulation in which case the treatment is dangerous due to a high risk of radiation induced lung injury. If the nuclear medicine lung scan is satisfactory, then the patient returns in 1-2 weeks for administration of the yttrium-labelled spheres to the tumour via the hepatic artery. Following the procedure, the femoral puncture site is usually compressed by hand or a specialised arterial closure device may be used.
Serious complications are rare but in some patients radiation hepatitis occurs due to excessive irradiation of the normal liver, which can lead to liver failure.
Possible restrictions to the use of SIRT
SIRT may not be possible in patients who have/had: Liver failure or severely abnormal liver function tests, ascites (fluid within the abdominal cavity), previous external beam radiotherapy to the liver, treatment with capecitabine (a chemotherapy agent) within the previous two months, or if capecitabine treatment is planned within 2 months of SIRT.
SIRT is not usually undertaken if there is evidence of extensive spread of tumour outside the liver.
SIRT would not generally be undertaken in patients where the extent of cancer within the liver is amenable to surgical resection.
_in_vessels_close_to_tumour_-_jpeg.jpg)
_-_jpeg.jpg)
Figure 6a – CT scan demonstrating liver metastases (arrows) involving right lobe and at the central region of liver (where branches of blood vessels and bile ducts join).
Figure 6b - Selective placement of embolisation coils (arrow) in vessels close to tumour.
Figure 6c - Angiogram taken during SIRT. Note blood supply of tumour in the right lobe of liver
Role of SIRT
The exact role of SIRT in the treatment of liver tumours is currently being established. SIRT as a first line therapy for patients with colorectal metastases is currently under investigation in the UK FOXFIRE trial 16 and the SIRFLOX trial in Australia. The results are awaited for further evidence regarding SIRT in colorectal liver metastases.
Treatment with SIRT is generally not regarded as a cure, but is thought to shrink the tumour when combined with chemotherapy more than just chemotherapy alone. The aim of treatment is to increase life expectancy and improve quality of life. On occasion, some patients treated with SIRT have had enough shrinkage of the liver tumour that surgical removal can be attempted.
Percutaneous Ablation Techniques / Radiofrequency ablation (RFA)
Another route to treat primary and secondary solid organ tumours is via direct ablation (using thermal energy to destroy cells and cause tumour necrosis.) This is an evolving technique and has been used in liver, renal and lung tumours with some success.
Liver ablation techniques
Percutaneous Ethanol Injection (PEI) was an early technique involving the injection of absolute ethanol (alcohol) directly into HCC lesions under ultrasound control and achieved satisfactory results in small tumours <3cm. [16] Other techniques that have been used include cryoablation (freezing of tumours), microwave ablation, and laser techniques, but radiofrequency ablation (RFA) remains the predominant technique. [17] RFA has been approved by NICE (National Institute of Clinical Excellence) for the treatment of unresectable HCC and colorectal hepatic metastases. [18]
RFA – Mechanism of action
RFA produces movement of ions in the tissue which results in heating and cellular death. Heating to a temperature of 60-100 oC results in almost immediate tissue damage.
RFA is based on producing tissue necrosis using a high-frequency alternating current that is delivered through an electrode placed in the centre of the tumour [19, 20]. Tissue necrosis begins as the temperature approaches 60°C, and RFA treatments often result in local tissue temperatures that approach or exceed 100°C, which result in tumour cell death.
It is possible to treat single tumours of up to 5 cm in diameter, and multiple tumours of <3cm diameter.
Technique of RFA
RFA may be performed either under sedation or general anaesthesia. The liver lesions will have been identified using either ultrasound (US) or computed tomography (CT), and the RFA procedure can be performed under either US or CT guidance, which is usually determined by the interventional radiologist prior to the procedure.
The procedure would normally be performed in the CT scanner or the interventional radiology suite. Once positioned upon the scanning table, the skin over the liver will be cleaned and sterilised and a sterile drape applied. Local anaesthetic is infiltrated into the overlying tissues and either sedation or general anaesthesia is required for pain relief during the procedure.
An insulated needle with an electrode at the tip is used which transmits high-frequency alternating current to the tumour tissue. The needle electrode is inserted into the tumour usually under ultrasound guidance with CT to confirm the final position.
Following ablation of the tumour, continued heating of the needle on withdrawal or “track ablation” avoids spreading of tumour cells.
Figure 7a – Small peripheral liver tumour in right lobe of liver (black arrow) pre-ablation
Figure 7b – Intraoperative CT image of RFA needle in position within lesion (white arrow)
Figure 7c – Post RFA CT image demonstrating tumour necrosis (white arrow)
Restrictions to the use of RFA
The following factors may mean that RFA cannot be performed:
Significant evidence of cancer outside the liver, invasion of bile ducts or major vessels, liver cirrhosis or active infection. Difficult to access lesions (may be sometimes necessary to perform RFA under open or laparoscopic (keyhole) surgery.)
Tumours that occupy >40% of the volume of the liver (the amount of liver left after RFA might not be sufficient to preserve liver function.)
Close distance to important structures like vessels and nearby organs (but open RFA may be possible.)
A relative contraindication may be if lesions are larger than 5 cm:
RFA should be used cautiously for lesions larger than 5 cm. One study suggests the use of open / surgical RFA for lesions larger than 5 cm. [21, 22]
Complications of RFA
Complications of RFA include haemorrhage, liver abscess, and heat injury to adjacent structures e.g. bowel and gallbladder. The use of “hydrodissection” (the injection of dextrose solution to push away other nearby organs) can be used to avoid local complications or injury to other structures.
Results of RFA in HCC, either alone or in combination with TACE have been encouraging.
Is RFA the preferred treatment?
Despite many published reports of RFA in HCC and liver metastases, large scale randomised trials comparing RFA, TACE and SIRT in the treatment of these tumours are still awaited. It is not easy to clearly state which treatment is best for each tumour size and distribution, and multidisciplinary discussion between oncologists, surgeons and interventional radiologists is the best approach to determining the best course of treatment.
NICE guidance
The National Institute for Clinical Excellence (NICE) is responsible for reviewing and recommending medical treatments and guidelines in the UK.
Guidance has been published with recommendations on RFA treatment in HCC and colorectal liver metastases.
Radiofrequency ablation of hepatocellular carcinoma (first published July 2003)
Radiofrequency ablation for the treatment of colorectal metastases in the liver (first published September 2004) – Consultation document
Selective internal radiation therapy for colorectal metastases of the liver: guidance (first issued September 2004)
New and future treatments
The future of interventional oncology treatments will be decided by the development of new technologies.
Chemoembolisation (TACE) allows the targeted infusion of chemotherapy agents to the actual tumour and avoids side effects to the rest of the body. At the moment, the liver is best suited to this due to its dual blood supply, but this is more difficult in other organs.
Development of targeted chemotherapies that have a specialized “tag” that allow the drugs to attach to tumour cells will allow chemoembolisation to be administered even more accurately.
Gene therapy is a new type of treatment designed to repair or replace damaged genes in cancer cells, causing them to die. Other forms of gene therapy aim to add 'toxic' genes into cancer cells. Yet another approach is to add genes that either sensitise a tumour to treatments such as chemotherapy or radiotherapy, or protect healthy cells from their side effects.
Gene therapy research is still at an early stage and at the moment gene-based treatments are not widely available for cancer patients.
High-intensity focused ultrasound (HIFU) is a new technique where high intensity ultrasound energy is focused into tumour tissue. Ultrasound at low energies is used for diagnostic purposes and HIFU uses high energy ultrasound under ultrasound or MRI guidance to target the energy to a specific position within the body. Tissue temperatures can rise to 65-85 degrees Celsius, which is high enough to cause tissue destruction. HIFU is performed without any incisions or surgery and is at the cutting edge of new radiological treatments. [23]
At present it is not available in the NHS and is only performed in a few centres in the UK, in the context of trials, but is a realistic prospect for the future.
Cost (NHS tariff and typical private range)
To be completed
References
- Stuart KE, Stadler ZK; Hepatocellular Carcinoma, Primary. Updated: Jan 12, 2009.
- Bosch FX, Ribes J, Diaz M, et al; Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004 Nov;127(5 Suppl 1):S5-S16. [abstract]
- Ryder SD. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (HCC) in adults, British Society of Gastroenterology (2003)
- Okuda K. Clinical presentation and natural history of hepatocellular carcinoma and other liver cancers. In: Okuda K, Tabor E, eds. Liver Cancer. New York: Churchill Livingstone, 1997:1–12.
- Nawaz Khan A, MacDonald S, Pankhania A, Sherlock D. Liver Metastases. Emedicine Radiology. http://emedicine.medscape.com/article/369936-overview
- El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008 May;134(6):1752-63. Review.
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359(4):378-90.
- Egawa H, Maki A, Mori K. Effects of intraarterial chemotherapy with a new lipophilic anticancer agent, estradiol-chlorambucil (KM2210), dissolved in lipiodol on experimental liver tumor in rats. J Surg Oncol.1990; 44:109-114.
- Konno T. Targeting cancer chemotherapeutic agents by use of lipiodol contrast medium. Cancer.1990;66:1897-1903.
- Kruskal JB, Hlatky L, Hahnfeldt P, et al. In vivo and in vitro analysis of the effectiveness of doxorubicin combined with temporary arterial occlusion in liver tumors. J Vasc Intervent Radiol.1993;4:741-748.
- Jacobson DR. Hepatocellular Carcinoma. EMedicine. Medscape.com, Feb 2009.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442.
- Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6-25.
- Khan AN, MacDonald S, Tam C-L, et al. Hepatic Chemoembolization. EMedicine, Medscape.com, April 2008
- Fiorentini G, Aliberti C, Turrisi G, Del Conte A, Rossi S, Benea G et al. Intraarterial hepatic chemoembolization of liver metastases from colorectal cancer adopting irinotecan-eluting beads: results of a phase II clinical study. In Vivo. 2007;21:1085-1091.
- Ryu M, Shimamura Y, Kinoshita T, et al. Therapeutic results of resection, transcatheter arterial embolization and percutaneous transhepatic ethanol injection in 3225 patients with hepatocellular carcinoma: a retrospective multicenter study. Jpn J Clin Oncol 1997;27:251–257.
- Bouza C, López-Cuadrado T, Alcázar R, Saz-Parkinson Z, Amate JM. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009 May 11;9:31. Review.
- National Institute for Health and Clinical Excellence.
- Curley SA, Izzo F. Radiofrequency ablation of primary and metastatic hepatic malignancies. Int J Clin Oncol 2002;7:72–81.
- Buscarini E, Savoia A, Brambilla G et al. Radiofrequency thermal ablation of liver tumors. Eur Radiol 2005;15:884–894.
- Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. 2007;10:38-46.
- Bilchik AJ, Wood TF, Allegra DP. Radiofrequency ablation of unresectable hepatic malignancies: lessons learned. Oncologist. 2001;6(1):24-33.
- http://info.cancerresearchuk.org/cancerandresearch
Links (to other useful sites)
NICE – National Institute for Clinical Excellence
Radiology Info – A US patient information site on radiological procedures
http://www.radiologyinfo.org/en/info.cfm?pg=chemoembol
Society of Interventional Radiology - A US patient information site on interventional radiological procedures
SIRTEX website – Information on manufacturer of SIRT spheres
Videos
Below are links to websites on “You Tube” that contain information on interventional radiological cancer treatments. These should be used for illustrative information, and the content is not necessarily approved by the British Society of Interventional Radiology.
RF - Ablation Tumours - http://www.youtube.com/watch?v=FcqAegf6q3w
Liver Cancer - http://www.youtube.com/watch?v=cKQETTaaiJc
SIR Therapy - http://www.youtube.com/watch?v=735nO2PAyCo
ChemoEmbolization (TACE) for Liver Cancer - http://www.youtube.com/watch?v=sSthh_0Y9qQ
Interventional Radiology: Chemoembolization Treatment at Stanford Hospital - http://www.youtube.com/watch?v=V32F_esruyI
RFA - Alternative Cancer Treatment - http://www.youtube.com/watch?v=7aB6cDM2wg4