Patients
-
- Angiography
- Angioplasty and Stenting
- Aortic Aneurysms
- Biliary Drainage and Stenting
- Carotid Artery Stenting
- Central Venous Access
- Colonic Stenting
- Fibroids
- Gastrointestinal Haemorrhage
- Gastrostomy
- Hepatic Malignancies
- Kidney Tumour Ablation
- Minimally Invasive Treatments for Vascular Disease
- Nephrostomy
- Oesophageal Stents
- Pelvic Venous Congestion Syndrome
- Percutaneous Nephrolithotomy
- Prostate Artery Embolisation PAE
- Pulmonary Arteriovenous Malformations
- PAE Patient Information Leaflet
- Ureteric Stenting
- Varicoceles
- Varicose Veins
- Vascular Malformations
- Vertebral Compression Fractures
- Vertebroplasty and Kyphoplasty
Pulmonary Arteriovenous Malformations
Author: Dr Iain Robertson, Consultant Interventional Radiologist, Gartnavel General Hospital , Glasgow.
Contents |
What are pulmonary arteriovenous malformations?
Arteriovenous malformations are abnormal connections between the artery (arterio) and vein (venous). When they occur in the lung they are termed pulmonary arteriovenous malformations (PAVM).
The abnormal connection usually causes increased blood flow through the area; the severity of this depends on the size and number of blood vessels involved.
Some people will have more than one PAVM; in more than half of patients with multiple PAVM this is associated with a condition called Hereditary Haemorrhagic Telangectasia.
What causes PAVM?
Pulmonary arteriovenous malformations are usually congenital.
The commonest association is Hereditary haemorrhagic telangiectasia (HHT). It is reported that at least a third of those with a single PAVM and at least half of those with multiple PAVM's have HHT .
What are the symptoms?
Most patients are asymptomatic.
PAVM are often detected during a CT scan done for other conditions or because of screening when a relative has Heriditary Haemorrhagic Telangectasia.
Increased blood flow through a PAVM can lead to-
Breathlessness: the blood flow short circuits the part of the lung that gives blood oxygen.
Stroke: the small blood vessels in the lungs act as a filter to prevent tiny blood clots from the veins entering the arteries and blocking the blood supply to organs. The risk is highest for the blood vessels going to the brain as they are some of the first branches. Patients with a patent PAVM have a risk of stroke of between 1-1.5% per year.
Heart failure: the short circuit means that the heart pumps harder to try to get more oxygen into the bloodstream. Most commonly patients need to have a number of PAVMs to develop heart failure.
Infections in the brain or at other sites: the lungs usually help to filter out small clots and bacteria. Because these bypass the lung in PAVM they can travel to sites in the arterial circulation
Bleeding: the abnormal blood vessels are often more fragile than usual vessels and under more pressure because of the short circuit. This is a relatively rare complication of PAVM.
How are PAVM diagnosed?
Screening for Heriditary Haemorrhagic Telangectasia includes test of oxygen levels when sitting up and lying down. A difference between these levels may indicate PAVM and can lead to further tests.
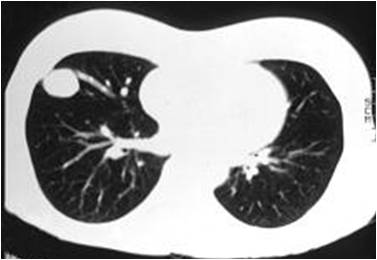
The diagnostic test most frequently used is CT scanning with X-ray contrast injection. (Figure 1).This scan is done as an outpatient and usually takes in total about 20 minutes. Modern CT scanners can scan the whole of the lungs in a single breath-hold and provide detailed images of the exact location and number of PAVM including the number and size of blood vessels involved in each PAVM. This information is essential in planning any treatment options.
Generally PAVM can be demonstrated if they are bigger than a few mm; in practice embolization isn’t usually possible for PAVM smaller than 3mm. Very small PAVM may not be detected and can cause abnormal blood flow though the risks of leaving such PAVM untreated are smaller than for larger lesions.
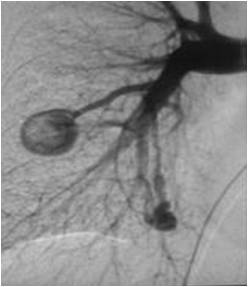 Angiography is now rarely needed as a diagnostic test (Figure 2).
Angiography is now rarely needed as a diagnostic test (Figure 2).
Occasionally, in very complex lesions angiography is still required as the radiologist can inject individual blood vessels and decide their contribution to PAVM and how to treat them.
How are PAVM treated?
The aims of treatment of PAVM are to block the PAVM and reduce the risk of stroke, heart failure and improve breathlessness.
The treatment options depend on the anatomy. The majority of PAVM are treated by a technique called embolization which blocks the feeding arteries to the PAVM. Rarely when there is a very extensive abnormality located in a single part of the lung this may be treated by a surgical operation to remove this area.
Embolization.
This procedure is performed under X-ray guidance in an specialized X-ray room called an angiographic suite or room. The procedure is usually performed by an interventional radiologist; a doctor who specialises in image guided treatment.
 Most PAVM are treated under local anaethesia with an injection in the groin used to numb the area where the catheter will be inserted. Sometimes patients may be given some sedation intravenously to help them relax; if you are feeling anxious then please ask if this is possible.
Most PAVM are treated under local anaethesia with an injection in the groin used to numb the area where the catheter will be inserted. Sometimes patients may be given some sedation intravenously to help them relax; if you are feeling anxious then please ask if this is possible.
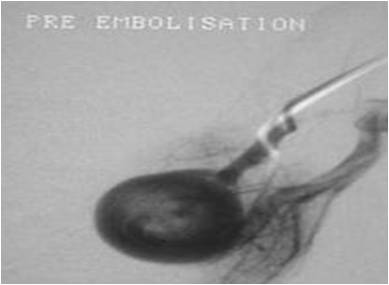
The groin is numbed with local anaesthetic and a catheter inserted into the main vein at the groin. The catheter can then be guided through the blood vessels into the area that the PAVM was identified on the CT scan. Contrast injection via the catheter outlines the blood vessels and helps guide the catheter into the correct vessels. There are hundreds of blood vessels in the lungs and it can take some time to enter the correct vessels; patience is required from both the patient and the radiologist! Once the catheter is in the feeding arteries a number of detailed pictures (often called runs) are taken to determine the exact size of the the blood vessels involved and plan where to leave the blocking material (Figure 3).
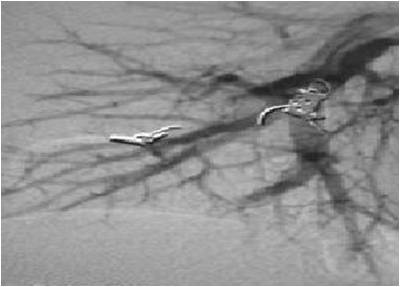
 The blocking material used will vary depending on the anatomy. The commonest material used are embolization coils; stainless steels springs that can be inserted through the catheter straightened out but which form a larger spiral when pushed out of the catheter (Figure 4).
The blocking material used will vary depending on the anatomy. The commonest material used are embolization coils; stainless steels springs that can be inserted through the catheter straightened out but which form a larger spiral when pushed out of the catheter (Figure 4).
 These vary in size from 2-20mm and it is usual to need several to block the feeding vessel. Increasingly, an alternative device called an Amplatz occluder is being used to treat larger PAVM. This device is effectively a cage of small wires held in a doughnut shape (Figure 5), this is delivered through the catheter compressed and expands when released from the catheter.The Amplatz occluder has the advantages that is is held onto the delivery wire and can be repositioned if the radiologist isn’t happy with the exact position and usually only one device is needed to block the artery.
These vary in size from 2-20mm and it is usual to need several to block the feeding vessel. Increasingly, an alternative device called an Amplatz occluder is being used to treat larger PAVM. This device is effectively a cage of small wires held in a doughnut shape (Figure 5), this is delivered through the catheter compressed and expands when released from the catheter.The Amplatz occluder has the advantages that is is held onto the delivery wire and can be repositioned if the radiologist isn’t happy with the exact position and usually only one device is needed to block the artery.
 Once the PAVM has been blocked the catheter is removed from the groin and the radiologist will press over the puncture site for five minutes or so to help the puncture seal (Figure 6).It is usual to have to lie flat for several hours, post treatment bed rest periods very from hospital to hospital but the local staff will let you know.
Once the PAVM has been blocked the catheter is removed from the groin and the radiologist will press over the puncture site for five minutes or so to help the puncture seal (Figure 6).It is usual to have to lie flat for several hours, post treatment bed rest periods very from hospital to hospital but the local staff will let you know.
What are the risks of treatment?
It is essential that you discuss your treatment with the staff involved in your care as they will be able to give a clear idea of risk and benefit for the individual anatomy of the your treatment. Risks will vary depending on the size and number of PAVM.
In general the risks of PAVM embolization are
Stroke or other artery blockage: during treatment the radiologist needs to work in the feeding artery of the PAVM. A small blood clot can form in the vessel or in the catheter used for treatment and this may pass through the abnormal communication into the arteries. In addition, there is a very small risk that the embolization material itself may pass through the communication despite every effort to make sure it is the correct size.
Damage to the blood vessels: as the catheter is guided through the blood vessels rarely damage to the vessels can occur. Most often this does not cause any symptoms but can rarely cause blockage or bleeding from the vessels.
Groin bruising: Some bruising around the puncture site is common and bruise in this area can take several weeks to fade.
What is the NICE guidance?
There is no NICE guidance available for this topic
